Alicia O. Hernandez-Castillo Rotational Spectroscopy (2)
Study of Succinimides and its Water Complexes with Rotational Spectroscopy
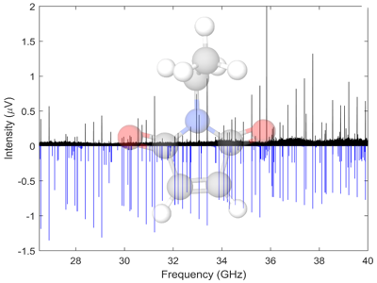
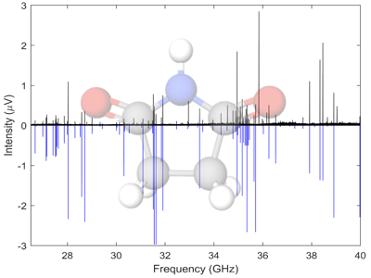
Succinimides are highly functionalized cyclic organics containing several H-bonding sites. We have acquired the rotational spectra of a number of succinimides and maleimides, these spectra have been acquired in the Ka band, 26.5-40 GHz, using the spectrometer at UC Davis. Both undergraduate students from the MolSpec lab and PhD students from the Kyle Crabtree’s group. Some molecules in this family show pseudorotation, a type of tunneling motion related to ring puckering which leads to additional splitting in the rotational spectrum.
This project also relies on the interplay of ab initio quantum mechanical calculations and experiments. We use various levels of electronic structure theory to acquire initial predictions for the rotational spectra, which then are used to assist with the interpretation of the experimental spectra.
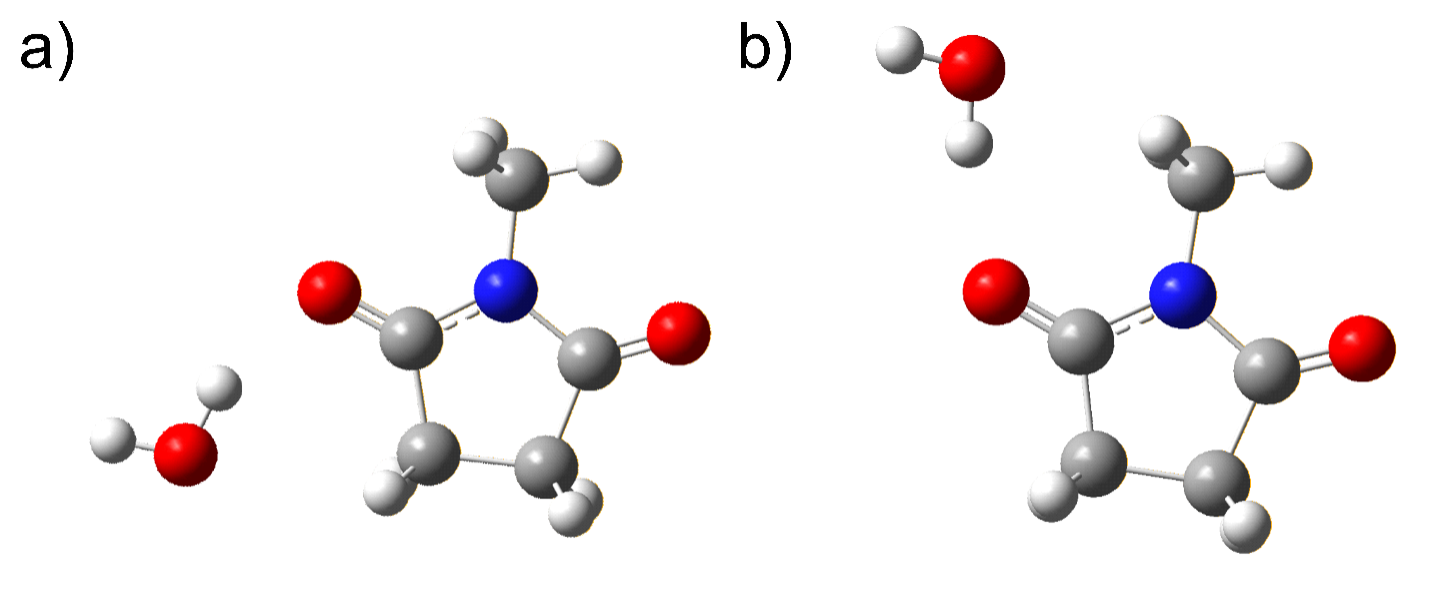
Now we are planning to study the water clusters of succinimides. These interactions with water are likely to be fascinating, as each water molecule can engage in up to four hydrogen bonds, two as acceptor and two as donor. Furthermore, the structures of the isolated succinimides themselves are likely to be modified by the interactions with water. In order to gain a truly molecular-scale understanding of aqueous solvation, we will form succinimide-(H2O)n clusters in the supersonic expansion and to study their structures using broadband microwave spectroscopy. In some sense, these clusters ‘fill the gap’ between the gas and condensed phases by building up successively larger microsolvated species in the supersonic expansion where we can study their structures in exquisite detail.
“Ka-band Rotational Spectroscopy of Succinimide and N-Chlorosuccinimide.” C.A. Dim, C. Sorrells, A.O. Hernandez-Castillo, , K.N. Crabtree, J. Phys. Chem. A, 2024, 128 (45), 9754-9762.
“A Tale of Two Tails: Rotational Spectroscopy of N-Ethyl Maleimide and N-Ethyl Succinimide.” L. York, C. Sorrells, C.A. Dim, K.N. Crabtree, A.O. Hernandez-Castillo, J. Phys. Chem. A, 2024, 128 (28), 5541–5547.