Harvey Mudd Researchers’ Discovery of Grain Splitting Published in Physical Review E
December 2, 2021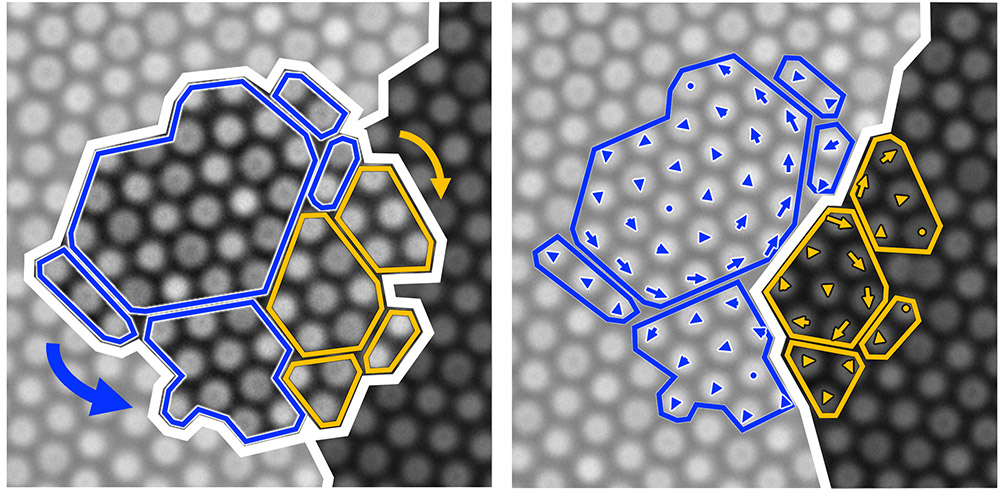
Harvey Mudd College Professor of Physics Sharon Gerbode and her team of research students co-authored a paper, “Grain splitting is a mechanism for grain coarsening in colloidal polycrystals,” which has been published as a “Letter” in Physical Review E. A research paper is selected as a “Letter” when its findings are deemed high-impact.
Gerbode and her team’s discovery of grain splitting paves the way for better models of crystal growth. According to Gerbode, the word “crystal” often evokes the image of endless rows of constituent particles neatly arranged in rows. However, most crystalline materials are composed of multiple grains, regions with rows oriented at different angles. The size and shape of grains determine various properties of the material, such as how strong it is, and how well it conducts electricity.
Gerbode’s research team discovered a surprising new method for how neighboring grains grow and merge. Until now, this grain-merging type of crystal growth has been understood in terms of two possibilities: either the smaller grain shrinks, while the other grows; or both neighbors can rotate to align their crystal rows and merge into a single crystal. But what happens when a grain has two neighbors that don’t agree on which way to orient their rows? Gerbode and her students discovered that in this situation, there is a third option: the grain can split apart and rotate in opposing directions to align with both neighbors. This “grain splitting” is more likely to occur in smaller grains, making this new mechanism particularly relevant in nanomaterials.
“The most exciting part of this discovery is that it presents a significant advancement in our understanding of how crystalline materials grow and age,” said Gerbode. “Until now, accepted theories have only offered two possibilities for how a crystal can merge with its neighbors, but we have discovered a third. This new mechanism will change how crystalline systems are modeled, offering better predictions for how a material changes over time. What’s shocking is that these theories have been around for so long, and no one had ever noticed that there was a third option, until now.”
The experimental discovery was made just before the pandemic hit, by then senior Maya Martinez ’20. The subsequent year was spent analyzing Martinez’s data and trying to understand this new grain splitting mechanism, an effort that was led by Anna Barth ’21. Martinez and Barth are co-first authors on the manuscript, along with Gerbode and several additional student co-authors from her lab: Cora Payne ’22, Christopher Gray Couto ’22, Izabela Joy Quintas ’22, Thorthong Soncharoen ’23, Nina M. Brown ’19 and Eli Joseph Weissler ’19.
“I am very proud that this manuscript, which combines experiment and theory, was produced entirely by my team of incredible undergraduate collaborators,” Gerbode said.
The practical applications of the discovery are significant.
“Imagine you have a nanocrystalline material, which has specific mechanical and electronic properties that have been carefully tuned by adjusting the size and placement of the grains,” explained Gerbode. “Over time, each grain will slowly evolve to merge with its neighbors, changing those prized properties—this process is called “aging.” If we want to have good estimates of how long that material will continue to have the properties we want, we need to have accurate models of the mechanisms that cause the grains to change.”
Until now, the models of aging relied on only two options: shrinking or rotating as a rigid object. Yet with these options, those models might paint too rosy a picture of how long such materials will last. Gerbode and her students’ discovery of a third option, grain splitting, and especially its importance in crystals with very small grains, means that the models can more accurately predict how long these nanocrystalline materials can be trusted to behave as expected, and importantly—when they need to be replaced.
